7 How many protons neutrons and electrons are in calcium 41. In this isotope.

How To Calculate The Number Of Protons Neutrons And Electrons Chemistry Youtube
No matter how many electrons or neutrons an atom has the element is defined by its number of protons.

. Solve any question of Structure of Atom with-. A How many neutrons are present in C-14 isotope of carbon. A C-14 isotope of carbon has mass number 14 and atomic number 6.
14 What is the electron number. 13 How many electrons are there. More than 999 of naturally occurring aluminium is 13 Al 27.
9 What has a charge of 2. Since a stable atom has a net charge of 0 we must have 20 electrons. It is also 20How many neutrons does calcium have20 neutronsN.
12 How do you find number of electrons. The number of protons plus the number of neutrons is 27. - Hence we can conclude that there are 13 protons 14 neutrons and 13 electrons are present in an atom of aluminium-27.
Number of neutrons depends on the isotopic form of aluminium. 9 electrons and 10 neutrons are present. Therefore the number of protons would be 19 and the number of neutrons would be 39-1920.
The periodic table is arranged in order of increasing atomic number so the number of protons is the element number. Well Z_textthe atomic number gives you PRECISELY the number of protons massive positively-charged nuclear particles and Z defines the IDENTITY of the atom And here Z28 and thus we got the element nickelthe second number 55 specifies the mass number of the atom and. The number of protons in Aluminium is 13.
It is also 20. So the atom contains 11 protons 11 electrons 12 neutrons. How many protons are present in an atom of Be-9.
Calcium is the 20th element with 20 protons. Tramwayniceix and 1 more users found this answer helpful. Fluorine is the element in question as its atomic number is 9.
Beryllium is a chemical element with atomic number 4 which means there are 4 protons in its nucleus. The one written below would represent the atomic number of the atom and this represents the number of protons in the atom. Since this atom has 9 protons then its atomic number will be 9.
How Many Protons And Electrons Does Calcium Have20 protonsHow many protons electrons and neutrons does calcium haveCalcium is the 20th element with 20 protons since the number of protons directly changes the element itself. 18 What is the full form of. If you have mass number and atomic number subtract the atomic number from the mass number to get the number of neutrons.
If we look on the periodic table atom number 9 is called fluorine. Its atomic number is 9 while its atomic mass is 19 atomic mass units. C How many electrons can be filled in the third orbit of an atom at the maximum.
Since a stable atom has a net charge of 0 we must have 20 electrons. This means every atom of oxygen has 8 protons. The mass number is 40.
For example a fluorine atom has 9 protons and 10 neutrons. The number of neutrons will be the same as the number of protons. Answer 1 of 2.
16 How do you solve protons neutrons and electrons. In fact its actually possible to have an atom consisting of only a proton ionized hydrogen. 8 How many protons neutrons and electrons does mg2 have.
Hence the number of neutrons will be 27 - 13 14. 16 How many electrons can n 4 have. 15 How do you find the number of neutrons in an ion.
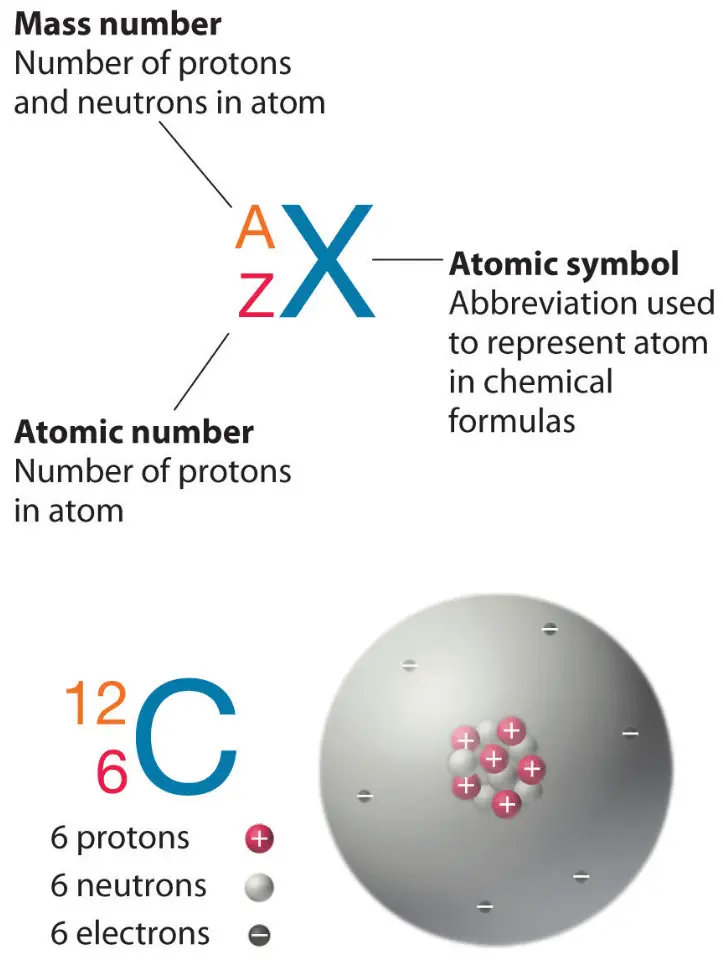
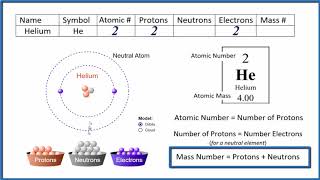
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. Mass number atomic number number of neutrons. 8 electrons 8 neutrons 6 protons.
So we can say that its atomic weight is 19 or its mass number is 19. 14 What is the number of protons for K. As we know that mass number number of protons number of neutrons.
17 How many electrons are there in the third shell of the atom with atomic number 23. What has 12 protons and 12 neutrons. 13 How do you find the number of positive ions.
What atom has 9 protons in its nucleus. That means the number of neutrons is 271314. How Many Protons Neutrons And Electrons Are In CalciumCalcium is the 20th element with 20 protons.
So for this case mass number would be 39 and the atomic number would be 19. The 27 means the atomic mass is 27. Atomic mass number is equal to sum of protons and neutrons.
Silicon has 14 protons 14 neutrons and 14 electrons. 11 How many electrons are in the 4th energy level. 11 What does Z mean in Chem.
5 How many neutrons does calcium have. The atomic number is the number of electrons as well as the number of protons whereas the atomic mass is the number of neutrons and number of protons. The atomic number is the same as the number of protons so since silicons atomic number is.
The atomic number is 20 40-20 20. The number of neutrons will be the same as the number of protons. B How many protons does He 2 ion possess.
But protonselectrons 199neutrons 19-9neutrons therefore there are 9 electrons and 10 neutrons. 15 What is the atomic mass of K. 4 How many protons and electrons are present in a calcium atom.
There are 4 protons in 9Be. 12 How do you calculate an ion. 17 What kind of ion is Na.
Protons and Neutrons in Beryllium. Since a stable atom has a net charge of 0 we must have 20 electrons. Of protons Atomic no.
9 What is the proton number of calcium. 6 How many protons neutrons and electrons does calcium 44 have. All isotopes and ions of the same elements will have the same number of protons regardless of the difference in the.
10 How many protons does be 2 have.

The Structure Of The Atom Boundless Chemistry
Magnesium Protons Neutrons Electrons Electron Configuration
How To Find The Number Of Protons Electrons Neutrons For Helium He Youtube
0 Comments